
Molecular engineering enables durable antiviral, antibacterial cotton
In recent decades, researchers have investigated various approaches to incorporating antiviral and antibacterial properties into cotton textiles due to their universal
Frequent outbreaks of epidemics in recent years, including influenza, gastroenteritis, tuberculosis, pneumonia and particularly the coronavirusdisease 2019 (COVID-19), have claimed numerous human lives worldwide. These diseases can spread through human activities where clothing and other textiles are primary vectors for the culture and transfer of viral and bacterial species. In recent decades, researchers have investigated various approaches to incorporating antiviral andantibacterial properties into cotton textiles due to their universal use.Cotton textiles are composed of hierarchical microstructures that consist of cellulose molecules, which are polymers derived from d-glucosemonomers via the linkage of β-(1,4) glycosidic bonds.
Figure 1: The structure and performance of the Cu-IT. a)
Schematic showing the structure of the Cu-IT and the proposed mechanism of its antiviral andantibacterial properties. The coordinated copper ions within the cellulosefibres can interact with the viral genome and prevent the virus from replicating.Additionally, the copper ions cause bacterial cell membrane rupture, resulting in a loss of the membrane potential and cytoplasmic content; they can alsoinduce the production of ROS, leading to denaturation of DNA and cell death. b) A comparison of the performance of the Cu-IT to that of unmodified and surface-modified cotton textiles.
Cellulose polymers are typically biosynthesised from cellulose synthase complex (CSC) and form as elementary fibrils of approximately 1.5–3.5 nm indiameter. These elementary fibrils can further self-assemble into larger bundles, termed cellulose nano fibres, with a nominal cross-sectional size of ~10 nm, which ultimately form microfibres with diameters of decadal micrometres. However, current mainstream strategies for producing antiviral and antibacterial textiles only physically load antiviral and antibacterial additives onto the fabric and do not fully leverage the material’s molecular structures. For example, a range of antiviral and antimicrobial agents, such as organic compounds (for example, quaternary ammonium compounds, triclosan, polyhexamethylenebiguanide and N-halamines), synthetic or natural polymers (for example, polypyrrole, chitosan and specific natural dyes), graphene materials and metal-based materials (for example, copper, silver and zinc,and their oxides and salts), have been physically incorporated into textiles. In a specific example, cotton textiles coated with metalliccopper, Cu2O and CuO nanoparticles were shown to exhibit high antiviral and antibacterial effects.
However, these additives are typicallyintroduced into the textiles via methods such as vapour deposition,evaporation, sputtering and spraying, which raises concerns about thecoating durability against wearing and washing due to low adhesivity, weak mechanical strength and limited bonding ability (for example, weak electrostatic interactions). In addition, the high expense anddifficulty of scaling up prevent the wide application of these methods. Here we introduce a new strategy of fabricating antimicrobial cotton textiles based on a fundamentally different principle of incorporating copper ions into the cotton structure at the molecular level, utilising strong coordination bonding between copper ions and the cellulosemolecules. This new approach can produce antiviral and antibacterial cotton textiles that are wearable and washable in a scalable and cost-effective manner, enabling practical application in everyday use.
The method employs a strategy of disrupting the hydrogen-bonding network interconnecting the cellulose chains, followed by diffusing Cu(II) ions into the swollen cellulosic materials, allowing them to coordinate with the hydroxyl groups on the cellulose chains and form a stable copper ion–cellulose complex (Figure 1a). The two processes are accomplished via a one-pot reaction by soaking a cotton textile in a Cu(II)-saturated NaOH solution. The coordination bonding between copper ions and their neighbouring cellulose chains makes this copper ion-textile (Cu-IT) highly stable in air and water, and durable against abrasion. The copper ions can interact with viral genomes and inhibit virus replication, and cause contact killing of bacteria and fungi by rupturing cell membranes and inducing reactive oxygen species (ROS).
Cu-IT also shows better mechanical properties, with a ~23 per cent increase in tensile strength compared with unmodified textiles, which is due to the role of the copper ions as ‘cross linkers’ between the cellulose molecular chains. As a proof-of-concept, we show that Cu-IT exhibits high antiviral and antibacterial activities against tobacco mosaic virus (TMV), influenza A virus (IAV), and Escherichia coli, Salmonella typhimurium, Pseudomonas aeruginosa and Bacillus subtilisbacteria. Additionally, the fabrication of Cu-IT is simple, scalable and cost-effective, and can be applied to different types of cotton fabrics (Figure 1b). This methodology may provide a renewed understanding of the potential of cellulosic materials, adding new dimensions to their application, including improved public health and hygiene.
Material synthesis and characterisation
The Cu-IT fabrication process only requires a simple set-up and inexpensive chemicals. First, a piece of cotton textile was immersed in a blue Cu(II)-saturated aqueous NaOH solution until no further colour change was observed in the fabric. Then, the blue textile was taken out
and washed with deionised (DI) water to remove residual NaOH and excess Cu(II) ions. Finally, the textile was dried, ready for further use (see Methods for more details). Visual inspection of the unmodified textile and Cu-IT (20 cm × 8 cm) is shown in Fig. 2a,b. The control parameters content increased with the reaction time and plateaued at 12.64 wt per cent after soaking for 1 day.
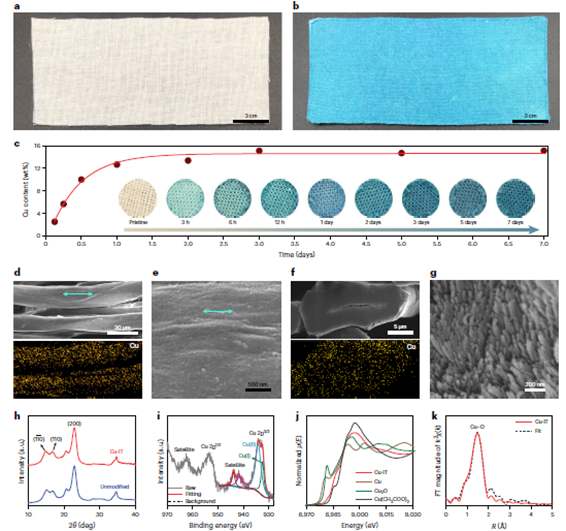
Figure 2: Characterisation of the Cu-IT. a,b, Photographs of the unmodified cotton textile (a) and the Cu-IT (b) (20 cm × 8 cm). c, The copper contents in the Cu-IT samples produced using different soaking times. Insets: corresponding photographs of the textile samples. d, SEM micrograph of the cotton microfibers within the Cu-IT and the corresponding copper elemental mapping. e, Higher magnification SEM micrograph of the Cu-IT. f, Cross-sectional SEM micrograph of a Cu-IT microfibre and the corresponding copper elemental mapping. g, Higher-magnification cross-sectional SEM micrograph of elementary fibrils that constitutes the microfibres of the Cu-IT. h, X-ray diffraction profile of the unmodified textile and Cu-IT. i, XPS Cu 2p spectrum of the Cu-IT. j, Cu K-edge XANES spectra of the Cu-IT; spectra of Cu, Cu2O, and Cu(CH3COO)2 standards are shown for comparison purposes. k, R-space EXAFS spectrum of the Cu-IT and the corresponding fitting curve. FT, Fourier transform
Scanning electron microscopy (SEM) micrographs reveal that the Cu(II)-saturated NaOH solution does not change the morphology of the textiles (Supplementary Figure 5). Importantly, no particles are observed on the surfaces of the cellulose microfibres (Figure 2d) and nanofibres (Figure 2e) of the Cu-IT, indicating the absence of copper salts or oxides. Energy dispersive spectroscopy (EDS) has also been used to confirm the uniform distribution of copper throughout the cellulose micro fibres (bottom of Figure 2d and Supplementary Fig. 6). Additionally, no sodium is observed in the EDS spectrum in Supplementary Fig. 6, suggesting that NaOH has been thoroughly removed during the washing process. Finally, the cross-sectional morphology and copper mapping of the microfibres revealed by SEM imaging and EDS analysis (Figure 2f,g and Supplementary Figure 7) confirm the well-preserved microstructures of the cotton fibres and the even distribution of the copper ions throughout the fibres. Our strategy of incorporating copper ions into the microfibers leads to a more homogeneous distribution of copper compared with prior antimicrobial cotton fabrics, in which copper particles were deposited on the fibre surfaces. Therefore, we hypothesise that the copper ions of the Cu-IT should be more stable.
The copper insertion mechanism has been further studied. Two processes occur with the textiles during soaking in the Cu(II)-saturated NaOH solution. The alkaline environment effectively disrupts the existing hydrogen-bonding networks, resulting in a swollen cellulose matrix. Next, the Cu(II) ions can diffuse into the cellulose crystals and the gaps between crystals to coordinate with oxygen atoms of the hydroxyl groups on the cellulose chains. Note that the crystal structure of cellulose also changes from cellulose-I32 with parallel chain packing to cellulose-II with antiparallel packing33 during the first process.
Such molecular conformations and packing modes provide optimal geometries for copper coordination to form a new crystal structure of Na-cellulose II(Cu), as verified by X-ray diffraction analysis (Supplementary Fig. 8). After removing NaOH by washing with DI water
and drying, the expanded Na-cellulose II(Cu) lattices collapse and the cellulose-I lattices are recovered, which is shown by the resemblance between the X-ray diffraction patterns of the unmodified textile and Cu-IT (Figure 2h); additionally, no signature diffraction peaks associated with new crystal structures are observed.
The copper ions, however, are trapped between the unit cells of cellulose crystals, which we confirmed by two major experimental observations. First, the results from X-ray photoelectron spectroscopy (XPS) indicate the presence of copper species in the Cu-IT. The Cu 2pXPS spectrum of the Cu-IT (Figure 2i) shows a Cu 2p3/2 peak at 933.4 eV and an apparent satellite peak at 943 eV, indicating a mixed Cu(I) and Cu(II) state36,37. The appearance of a small amount of Cu(I) may be attributed to the weak reducing ability of cellulose38,39. We also note that a sodium signal is not detected (Supplementary Fig. 9), consistent with the EDS results of the Cu-IT (Supplementary Fig. 6), confirming that NaOH has been completely removed. Second, the coordinated state of copper ions in the Cu-IT textile was verified by X-ray absorption spectroscopy (XAS).
The Cu K-edge X-ray absorption near-edge structure (XANES) spectrum of the Cu-IT (Figure 2j) shows a broadened characteristic Cu(II) signature. A first-shell Cu–O bonding featuring a distance of 1.93 A and a copper coordination number of 4.0 were determined by fitting the extended X-ray absorption fine structure (EXAFS) spectrum of the Cu-IT (Fig. 2k). These spectroscopic findings, along with the results from calorimetric experiments and macroscopic properties such as the stable colour, indicate that copper ions are trapped in the cellulose matrix and stabilised via coordination bonding.
Antiviral and antibacterial activity
The antiviral and antibacterial properties of the Cu-IT have been tested (Fig. 3a). TMV and IAV were used as model viruses, and E. coli, S. typhimurium,P. aeruginosa and B. subtilis as model bacteria. The viral or bacterial strains were first incubated in the presence of the unmodified cotton textile control or Cu-IT (both textiles were sterilised before use). Then, the viruses and bacteria were inoculated on appropriate media to test the viral infectivity and bacterial viability. To assess the infectivity of TMV, one half of a Nicotianatabaccum leaf was inoculated with the Cu-IT-treated TMV and the other half with the unmodified textile-treated control. The number of lesions on the leaf after 5 days of plant growth was counted as a measure of the TMV infectivity. Meanwhile, to assess the infectivity of IAV, the textile-treated IAV solutions were inoculated on Madin–Darby canine kidney (MDCK) cells, and
plaques formed on the MDCK cell monolayer after 3 days of incubation were counted. For the antibacterial assessment, cell viability was measured by replicate plating of bacterial cultures (treated with the unmodified textile or Cu-IT) onto Luria–Bertani broth (LB) agar, and the agar plates were then counted for colonies after incubation overnight (see Methods for details).
The Cu-IT shows excellent antiviral activity against TMV. Figure 4b shows a photograph of the inoculated (with an initial TMV concentration of 500 ng ml−1, a highly infectious dose) N. Tabaccum leaves (after 5 days); the leaf on the left was inoculated with TMV being treated using Cu-IT or unmodified textile for 3 h, and the one on the right with TMV being treated for 24 h. No lesions are observed on the halves inoculated with Cu-IT-treated TMV and a 3 h treatment is sufficient for the Cu-IT to take effect (left leaf in Figure 3b), while a large number of lesions are observed on the halves inoculated with the unmodified textile-treated TMV and the lesion count is higher for TMV treated for 24 h. A quantitative analysis of the lesion count variation versus the initial TMV solution concentration and of the time for unmodified textile or Cu-IT treatment (3 and 24 h) is shown in Figure 3c. The N. tabaccumleaves inoculated with Cu-IT-treated TMV yield zero counts under all conditions. These results strongly indicate that the TMV infectivity can be effectively inhibited after exposure to the Cu-IT for a period as short as 3 h. Considering that TMV shows very high stability under various conditions, the Cu-IT demonstrates strong potential for use as an antiviral material with high efficacy against a broader range of virus strains.
The inhibitory effect of Cu-IT on IAV using a plaque assay has also been evaluated (Figure 3d). When the Cu-IT was incubated with a low concentration of IAV solution (~3 × 104 p.f.u. ml−1), the infectivity reduced significantly, 567-fold lower than the IAV solution incubated with no textile. In contrast, IAV incubated with the unmodified textile also resulted in a decrease in infectivity, but far less (4-fold). Moreover, when the Cu-IT was incubated with a high concentration of the IAV solution (~3 × 106 p.f.u. ml−1), no plaque was found, while a low decrease of infectivity (22.5-fold) was measured for the unmodified textile. It was noted that the result of 60 p.f.u. ml−1 in the case of a low concentration of IAV solution incubated with Cu-IT was caused by either cross-contamination or was a stochastic effect due to very low virus titre, as we retested the Cu-IT with and without wash by focus assay and did not find any foci. These results demonstrate the Cu-IT’s superior inhibitory activity against IAV.
The colony numbers for the Cu-IT-treated bacterial cultures (for all four strains: the Gram-negative bacteria E. coli, S. typhimurium and P. aeruginosa, and the Gram-positive bacteriumB. subtilis) were significantly lower than those for the unmodified textile-treated cultures (Fig. 3e). We quantified these results using colony-forming units (c.f.u.)counts (Fig. 3f) and found the viable cell count in the Cu-IT-treated cultures of E. coli, S. typhimurium, P. aeruginosa and B. subtilis were 1,000,000-fold, 8-fold, 10,000,000-fold and 40,000-fold lower than those in the unmodified textile-treated cultures. Note that varied bacteriostatic activities were observed for the four bacterial strains, which may be due to the differences in their cell membrane structures as well as their response to copper-induced ROS. However, detailed mechanistic studies are outside the scope of the current contribution. Further, comparisons have been made with the antiviral and antibacterial performance of the Cu-IT with that of various commercial antimicrobial materials and confirmed Cu-IT efficacy.
To study the biocompatibility of the Cu-IT with human skin, we performed a cytotoxicity assessment using artificial perspiration on human dermal fibroblasts. The results demonstrate that the Cu-IT doesnot cause cytotoxicity due to ions produced from the contact between Cu-IT and human perspiration. In summary, the observed antiviral and bacteriostatic properties suggest that the Cu-IT has high application potential in personal, clinical and medical environments, although follow-on toxicity analysis utilising mammalian cells and tissues will ensure safe implementation.
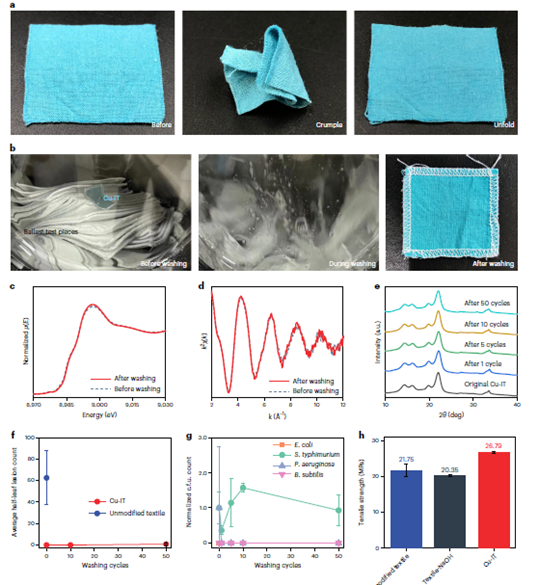
Figure 4: The mechanical properties and stability of the Cu-IT. a, Photographs showing the Cu-IT maintaining its shape after crumpling and unfolding. b, Photographs of a Cu-IT being washed based on the international standard ISO 6330-2012. c,d, The Cu K-edge XANES (c) and k-space EXAFS (d) spectra of the washed Cu-IT sample. e, X-ray diffraction patterns of the Cu-IT samples after different washing cycles. The Cu-IT samples were prepared after 1, 5, 10 and 50 washing cycles, and the washing time for every cycle was 15 min. f, Average half-leaf lesion counts of the leaves that had been inoculated with different TMV solutions. TMV solutions with an initial concentration of 500 mg ml−1 were pre treated with the washed Cu-IT for 3 h. The error bars represent the standard deviation of half-leaf lesion count. g, c.f.u. counts measured from the LB agar plates after the inoculation and overnight incubation of the washed Cu-IT samples treated with cultures of E. coli, S. typhimurium, P. aeruginosa and B. subtilis bacteria. The Cu-IT samples were prepared after different washing cycles and the c.f.u. counts are normalised to that of the unwashed Cu-IT sample. The error bars represent the standard deviation of normalised c.f.u. count. h, The tensile strength of unmodified cotton textile, textile treated with 10 per cent NaOH (Textile-NaOH) and Cu-IT. The error bars represent the standard deviation of tensile strength.
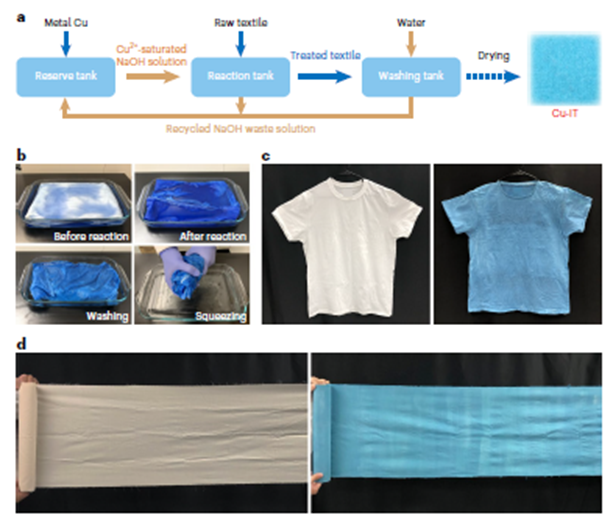
Figure 5: Scalable production of the Cu-IT. a, The fabrication process of the Cu-IT using recycled NaOH solution. b, Photographs showing the fabrication process of a Cu-IT T-shirt. c, Photographs of the pristine cotton T-shirt (left) and the Cu-IT T-shirt (right). d, Photographs of the pristine unbleached cotton cloth (left) and the Cu-IT cloth (right).
Mechanical properties and stability
We also assessed the mechanical properties and washing stability of the Cu-IT. The textile could be folded, crumpled and unfolded without issue, showing general characteristics comparable to those of unmodified textiles (Figure 4a), which we attribute to the well-preserved structures of the cellulose microfibres and macroscopic material integrity during treatment by the Cu(II)-saturated NaOH solution (Figure 2d–g and Supplementary Figure 5).
To test the material’s washing stability in water with detergent, a piece of Cu-IT was washed and dried according to the international standard ISO 6330-2012 (Figure 4b), with no apparent changes of colour or decreased integrity observed. We further verified that the coordinated structures were maintained using XAS (Figure 4c,d) and X-ray diffraction (Figure 4e). The Cu K-edge XANES and EXAFS spectra of the Cu-IT before and after washing were almost identical (the lines are essentially superimposed), and the X-ray diffraction profiles show nearly the same diffraction patterns. Furthermore, we measured the copper concentration in the wash wastewater from a modified (non-ISO) wash test (Supplementary Note 4) and, using a relationship between leached copper over time, estimated that the Cu-IT should endure thousands of washing cycles before reaching its copper half-life time (when the copper content in the Cu-IT decreases to half of its original value. This is longer than the typical lifespan of 200 wash–use cycles for cotton fabric. It has also been further assessed that the antiviral and antibacterial performance of the washed Cu-IT samples and found the antiviral
(Figure 4f) and antibacterial (Figure 4g) activities generally did not decay after repeated washing, suggesting textile reusability. The data for S. typhimurium seemed to indicate that after the first wash, the potency was reduced. This suggests there may be bacterial strain selectivity in antibacterial efficacy, but this would certainly be understandable and would warrant additional study prior to commercialised use.
No structural change was observed in the X-ray diffraction profiles of the Cu-IT stored for over 1 yr under ambient conditions. Additionally, we confirmed the good stability of Cu-IT against ultraviolet, heat and sweat. To investigate the durability of Cu-IT against abrasion during normal wear use, we performed abrasion-resistance tests on the Cu-IT and unmodified textile. After the abrasion tests, no apparent decrease of integrity was observed in the Cu-IT, while a rupture of the fibres occurred for the unmodified textile. Additionally, the Cu-IT maintained its copper content after abrasion, indicative of the even distribution of copper ions throughout the fibres, which should ensure excellent antiviral and antibacterial performance during everyday use.
Uniaxial tensile tests were performed to quantify the mechanical performance (Figure 4h). The tensile strength of Cu-IT was 26.79 MPa, which is ~23 per cent higher than that of the unmodified textile (21.75 MPa). In addition, cotton textile treated solely with aqueous NaOH solution, a process also known as mercerization, showed the lowest strength of 20.35 MPa, which may be attributed to the decrease of the degree of polymerisation of the cellulose polymers caused by the concentrated NaOH. Interestingly, the fractureproperties of the Cu-IT are different to those of the unmodified textile.
The fracture area of the Cu-IT is compact with a granular-type fracture, while that of the unmodified textile is loose with a fibrillary-type fracture. These observations demonstrate that the copper coordination within the microfibres improves the mechanical properties of the Cu-IT and highlights the critical role of copper ions in stabilising the secondary structure of cellulose.
Demonstration of scalable production
We suggest the three-step process (Figue 5a) for fabricating the Cu-IT is scalable using a roll-to-roll production method, which comprises the preparation of Cu(II)-saturated NaOH solution, coordination between Cu(II) ions and cellulose molecules via simple soaking, and finally, washing and drying. In addition, NaOH solution can be recycled, and metal copper is the only consumed raw material, making the process cost-effective and sustainable. Figure 5b shows an example of the fabrication scale-up, where a Cu-IT T-shirt was produced from a commercially available cotton T-shirt. The original cotton T-shirt was placed in a 300 mm × 200 mm × 30 mm container filled with Cu(II)-saturated NaOH solution (Supplementary Fig. 22) and soaked for ~7 days until the colour turned blue. After washing and drying, the resultant Cu-IT T-shirt (Figure 5c) exhibited well-preserved physical properties but slight shrinkage, which may be due to the alkaline solution treatment and the copper-ion coordination. As another example, a roll of Cu-IT cloth 35 cm wide and 280 cm long was prepared from unbleached cotton cloth using the same method (Figure 5d). It is worth also noting the inherent colour of the Cu-IT is similar to personal protective equipment (PPE) that is commonly used in healthcare settings. Thus, it renders another advantage of mitigating the environmental impact associated with the dyeing process. Altogether, this highly scalable, low-cost and eco friendly fabrication process endows the Cu-IT with great potential for practical use.
Conclusions
We have demonstrated a simple, cost-effective method for efficiently fabricating antiviral and antibacterial textiles by incorporating copper ions into cotton textiles at the molecular level. We showed that copper ions diffuse into the cotton textiles under alkaline conditions and coordinate with oxygen atoms of the hydroxyl groups on cellulose molecules and remain stabilised in the cellulose matrix after washing the textile to a neutral state. The Cu-IT shows high antiviral activity against the TMV and IAV and antibacterial activity against E. coli, S. typhimurium, P. aeruginosa and B. subtilis due to the sterilisation and disinfection efficacy of copper. The Cu-IT material is also stable and reusable and can sustain repeated washing and wearing. In addition, the coordination bonding enhances the mechanical strength of the modified cotton textile. This fabrication method is highly scalable and shows great potential for mass production of antiviral and antibacterial textiles used in household products, public facilities and medical settings.
References
- Kozel, T. R. & Burnham-Marusich, A. R. Point-of-care testing for infectious diseases: past, present, and future. J. Clin. Microbiol.55, 2313–2320 (2017).
- Sohrabi, C. et al. World Health Organization declares global emergency: a review of the 2019 novel coronavirus (COVID-19). Int. J. Surg. 76, 71–76 (2020).
- Amanat, F. & Krammer, F. SARS-CoV-2 vaccines: status report. Immunity 52, 583–589 (2020).
- Sidwell, R. W., Dixon, G. J. & Mcneil, E. Quantitative studies on fabrics as disseminators of viruses: I. Persistence of vaccinia virus on cotton and wool fabrics. Appl. Environ. Microbiol. 14, 55–59 (1966).
- Dixon, G. J., Sidwell, R. W. & Mcneil, E. Quantitative studies on fabrics as disseminators of viruses: II. Persistence of poliomyelitis virus on cotton and wool fabrics. Appl. Environ. Microbiol. 14, 183–188 (1966).
- Sidwell, R. W., Dixon, G. J., Westbrook, L. & Forziati, F. H. Quantitative studies on fabrics as disseminators of viruses: IV. Virus transmission by dry contact of fabrics. Appl. Environ.Microbiol. 19, 950–954 (1970).
- Gerba, C. P. & Kennedy, D. Enteric virus survival during household laundering and impact of disinfection with sodium hypochlorite. Appl. Environ. Microbiol. 73, 4425–4428 (2007).
- Katoh, I. et al. Potential risk of virus carryover by fabrics of personal protective gowns. Front. Public Health 7, 121 (2019).
- Ansell, M. P. & Mwaikambo, L. Y. in Handbook of Textile Fibre Structure Vol. 2 (eds Eichhorn, S. J. et al.) 62–94 (Woodhead, 2009).
- Hu, L. et al. Stretchable, porous, and conductive energy textiles. Nano Lett. 10, 708–714 (2010).
About the authors:
M Manoj Prabagar, Dr N Gokarneshan, A Jothimanikandan, P Periyasamy and Dr KC Karunakaran are from the Department of Textile Chemistry, SSM College of Engineering, Komarapalayam, Tamil Nadu.




